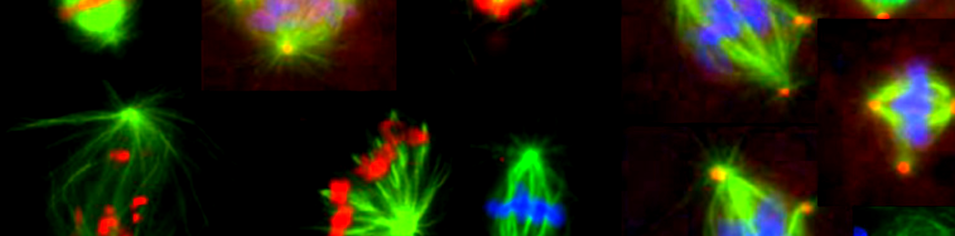
Background
Every living thing renews itself by a process of cell division. In order to produce two new identical cells from one existing cell, the genetic material, the chromosomes, must first be replicated and the volume of the cell must increase. During the process defined as cell division itself, these duplicated chromosomes align in the centre of the cell before one copy of each chromosome is moved to opposite sides of the cell. Finally, the cell physically cleaves in two, usually at the centre of the cell, producing two daughter cells, each identical to themselves and to the original cell.
All cells use protein fibres termed microtubules order to faithfully divide. These fibres are made up of repeating units of a protein called tubulin that can be added or taken away from the ends of existing microtubules, allowing them to grow and shrink. Other proteins in the cell, called microtubule associated proteins (MAPs) are able to alter the properties of the microtubules, causing them to grow or shrink more quickly, to link existing microtubules to each other, or to link the ends of the microtubules to chromosomes or other structures in the cell. In this way, MTs can be organised into dynamic structures, capable of doing different things.
During cell division, new microtubule growth is initiated at distinct sites in the cell, including centrosomes, the mitotic chromatin and within the growing spindle itself; this latter by a recently described protein complex, termed Augmin. These populations of microtubules are then organised, polarized and focused into a bipolar spindle apparatus, a self-organising, self-regulating structure which is capable of exerting physical force on the chromosomes, equally dividing them between the newly forming cells.
Aim of the lab
Quite simply, we want to understand the morphogenesis of the spindle apparatus. Reductionist approaches have accumulated a list of proteins required for spindle assembly. However, viewing the mitotic spindle as a "machine" in the conventional sense does not accurately reflect the dynamic, responsive robustness intrinsic to this cellular structure. There is a growing realisation that to understand spindle function we need to understand how constituent proteins integrate to nucleate populations of microtubules, how these populations organise to bipolarity and how the spindle self-regulates itself against stochastic environmental perturbations.
Why fruit flies?
Drosophila melanogaster has proved to be an excellent system both for studying cell division, and for the isolation of proteins that function during mitosis. There are a number of other labs throughout the world working on distinct aspects of cell division in this model organism. It is a close-knit community of scholars who are open and enthusiastic about advancing the field as quickly as possible.
Why is this important?
Cell division underlies the proliferation of all life. A clear connection exists between aging, many chronic conditions, and the regulation of cell division, with improper chromosome segregation being a hallmark of old, cancerous and diseased cells. Although drugs have been developed that target proteins with essential roles in spindle formation, the complexity of the process and the robustness of spindle formation even following perturbation makes it difficult to accurately predict how therapeutics will affect an individual.
More widely, a general property of biological systems is their ability to self-organise. Through investigating the multi-level properties of mitotic spindles and their relationship to function using interdisciplinary strategies, we hope to better define how biological complexity results from the interactions between discrete biological entities.
The approach of the lab
The move towards quantitative techniques, driven by technological advances in physical science methods, coupled to the wealth of information accumulated within biology using high-throughput approaches, is now allowing us to measure and analyse sub-cellular processes with a degree of accuracy not previously possible. This provides the researcher with “real” data, which can be combined with statistical methods and powerful computational tools to build models of biological processes that can be tested. However, we believe it is important to view such analyses within the context of the biological process being studied. Humans are able to process visual information and relate it to their previous experiences and accumulated knowledge in a manner impossible for computers. Immersing oneself in the viewing experience can lead to a greater understanding of the way in which a biological process ‘works‘. Such experiential, intuitive biology has been practiced for centuries and has led to many of the great advances in biology, medicine and related disciplines. By combining the recently advanced quantitative techniques and historically valuable qualitative approaches, we hope to move towards our goal of understanding mitotic spindle formation more quickly than using one approach alone.
Research networks
Hiro Ohkura, University of Edinburgh, UK
David Sharp, Einstein College of Medicine, NY, USA
Jon Scholey, University of California, Davis, USA
Maurizio Gatti, Universita di Roma ‘La Sapienza‘, Italy
Maria Grazia Giansanti, Universita di Roma ‘La Sapienza‘, Italy
Silvia Bonaccorsi, Universita di Roma ‘La Sapienza‘, Italy
Charlotte Deane, Department of Statistics, University of Oxford, UK
Alison Noble, Department of Engineering, University of Oxford, UK
Every living thing renews itself by a process of cell division. In order to produce two new identical cells from one existing cell, the genetic material, the chromosomes, must first be replicated and the volume of the cell must increase. During the process defined as cell division itself, these duplicated chromosomes align in the centre of the cell before one copy of each chromosome is moved to opposite sides of the cell. Finally, the cell physically cleaves in two, usually at the centre of the cell, producing two daughter cells, each identical to themselves and to the original cell.
All cells use protein fibres termed microtubules order to faithfully divide. These fibres are made up of repeating units of a protein called tubulin that can be added or taken away from the ends of existing microtubules, allowing them to grow and shrink. Other proteins in the cell, called microtubule associated proteins (MAPs) are able to alter the properties of the microtubules, causing them to grow or shrink more quickly, to link existing microtubules to each other, or to link the ends of the microtubules to chromosomes or other structures in the cell. In this way, MTs can be organised into dynamic structures, capable of doing different things.
During cell division, new microtubule growth is initiated at distinct sites in the cell, including centrosomes, the mitotic chromatin and within the growing spindle itself; this latter by a recently described protein complex, termed Augmin. These populations of microtubules are then organised, polarized and focused into a bipolar spindle apparatus, a self-organising, self-regulating structure which is capable of exerting physical force on the chromosomes, equally dividing them between the newly forming cells.
Aim of the lab
Quite simply, we want to understand the morphogenesis of the spindle apparatus. Reductionist approaches have accumulated a list of proteins required for spindle assembly. However, viewing the mitotic spindle as a "machine" in the conventional sense does not accurately reflect the dynamic, responsive robustness intrinsic to this cellular structure. There is a growing realisation that to understand spindle function we need to understand how constituent proteins integrate to nucleate populations of microtubules, how these populations organise to bipolarity and how the spindle self-regulates itself against stochastic environmental perturbations.
Why fruit flies?
Drosophila melanogaster has proved to be an excellent system both for studying cell division, and for the isolation of proteins that function during mitosis. There are a number of other labs throughout the world working on distinct aspects of cell division in this model organism. It is a close-knit community of scholars who are open and enthusiastic about advancing the field as quickly as possible.
Why is this important?
Cell division underlies the proliferation of all life. A clear connection exists between aging, many chronic conditions, and the regulation of cell division, with improper chromosome segregation being a hallmark of old, cancerous and diseased cells. Although drugs have been developed that target proteins with essential roles in spindle formation, the complexity of the process and the robustness of spindle formation even following perturbation makes it difficult to accurately predict how therapeutics will affect an individual.
More widely, a general property of biological systems is their ability to self-organise. Through investigating the multi-level properties of mitotic spindles and their relationship to function using interdisciplinary strategies, we hope to better define how biological complexity results from the interactions between discrete biological entities.
The approach of the lab
The move towards quantitative techniques, driven by technological advances in physical science methods, coupled to the wealth of information accumulated within biology using high-throughput approaches, is now allowing us to measure and analyse sub-cellular processes with a degree of accuracy not previously possible. This provides the researcher with “real” data, which can be combined with statistical methods and powerful computational tools to build models of biological processes that can be tested. However, we believe it is important to view such analyses within the context of the biological process being studied. Humans are able to process visual information and relate it to their previous experiences and accumulated knowledge in a manner impossible for computers. Immersing oneself in the viewing experience can lead to a greater understanding of the way in which a biological process ‘works‘. Such experiential, intuitive biology has been practiced for centuries and has led to many of the great advances in biology, medicine and related disciplines. By combining the recently advanced quantitative techniques and historically valuable qualitative approaches, we hope to move towards our goal of understanding mitotic spindle formation more quickly than using one approach alone.
Research networks
Hiro Ohkura, University of Edinburgh, UK
David Sharp, Einstein College of Medicine, NY, USA
Jon Scholey, University of California, Davis, USA
Maurizio Gatti, Universita di Roma ‘La Sapienza‘, Italy
Maria Grazia Giansanti, Universita di Roma ‘La Sapienza‘, Italy
Silvia Bonaccorsi, Universita di Roma ‘La Sapienza‘, Italy
Charlotte Deane, Department of Statistics, University of Oxford, UK
Alison Noble, Department of Engineering, University of Oxford, UK